TIM-3 and Alzheimer’s treatment are emerging as a promising duo in the fight against Alzheimer’s disease. Researchers have discovered that the TIM-3 molecule, traditionally recognized as a checkpoint regulator in immune responses, plays a crucial role in the pathophysiology of Alzheimer’s. This innovative approach, inspired by immune system therapy previously successful in cancer treatments, seeks to unleash microglia from their inhibitory state, allowing them to clear toxic plaques from the brain. Early studies have shown that disabling TIM-3 can lead to cognitive improvement in mice models, offering hope for new therapeutic strategies. As the scientific community dives deeper, the potential of targeting TIM-3 in treating Alzheimer’s not only opens doors for enhanced cognition but could also reshape our understanding of neurodegenerative disease management.
The exploration of TIM-3 in combating cognitive decline associated with Alzheimer’s presents a novel therapeutic avenue. This immune checkpoint, known for its role in regulating immune responses, has now been identified as a pivotal factor in the brain’s battle against amyloid beta plaques. By focusing on immune system modulation, researchers aim to leverage strategies that enhance the brain’s natural defense mechanisms, much like those already utilized in oncology. The interplay between immune function and neurodegeneration suggests a multidimensional approach to Alzheimer’s treatment, where disrupting the TIM-3 pathway could lead to significant strides in memory restoration. As investigations continue, the integration of immune system therapies could redefine therapeutic landscapes for Alzheimer’s patients.
Understanding TIM-3 and Its Role in Alzheimer’s Disease
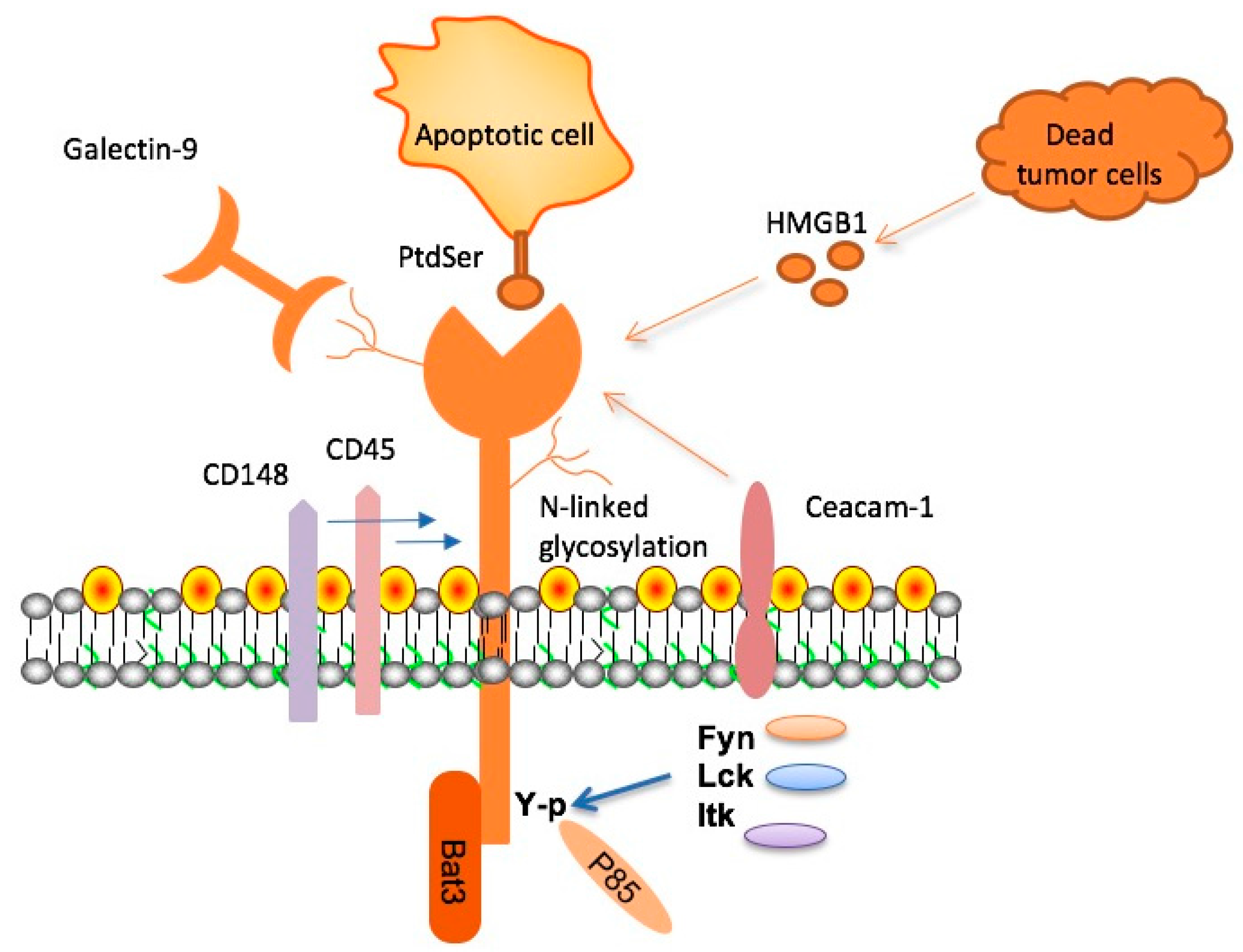
TIM-3, or T-cell immunoglobulin and mucin-domain containing-3, is identified as a critical checkpoint molecule in the immune system that has significant implications for Alzheimer’s disease. Research reveals that this molecule acts as an inhibitor, regulating the immune responses of microglial cells, the brain’s primary immune defenders. In patients with Alzheimer’s disease (AD), particularly those with late-onset forms, TIM-3 expression is notably heightened, contributing to a failure in clearing amyloid-beta plaques from the brain. This accumulation of plaques is a hallmark of Alzheimer’s and correlates with cognitive decline, making the understanding of TIM-3 essential for developing new therapeutic strategies.
By inhibiting TIM-3 in animal models of Alzheimer’s, researchers have observed a remarkable improvement in cognitive function. These findings indicate that removing TIM-3’s inhibitory effect allows microglia to actively engage in clearing the harmful amyloid plaques, thereby enhancing memory and cognitive abilities. Such discoveries underscore the molecule’s dual role in both maintaining immune homeostasis and potentially exacerbating neurodegenerative responses in Alzheimer’s pathology.
Immune System Therapy: A Novel Approach to Alzheimer’s Treatment
Innovative immune system therapies, akin to those employed in cancer treatment, present a promising avenue for Alzheimer’s disease management. Studies have shown that checkpoint inhibitors, which disrupt molecules like TIM-3, can rejuvenate the immune response, enabling the microglia to tackle and clear the amyloid plaques effectively. This approach not only seeks to restore cognitive function but also aims to mitigate the underlying pathology of Alzheimer’s.
Unlike traditional Alzheimer’s treatments that primarily target amyloid-beta or tau proteins through antibodies and other pharmacological means, the immune system therapy targets the malfunctioning immune responses themselves. By leveraging an understanding of the immune checkpoints, particularly TIM-3, researchers are developing therapies that not only halt plaque buildup but also encourage the brain’s self-cleaning mechanisms, opening a new frontier in Alzheimer’s disease treatment.
The Potential of TIM-3 Molecule in Treating Alzheimer’s Disease
The potential of targeting the TIM-3 molecule in Alzheimer’s treatment lies in its unique ability to manage the balance between necessary immune responses and potentially harmful overactivation. Recent studies indicate that by blocking the effects of TIM-3, microglia can regain their phagocytic capabilities, thus providing a pathway for removing the toxic plaques associated with Alzheimer’s disease. This not only has theoretical implications but has also demonstrated tangible improvements in cognitive tests performed on treated mice.
Moreover, the strategic manipulation of TIM-3 holds promise for developing less invasive treatments, such as administering anti-TIM-3 antibodies or small molecules that can selectively inhibit TIM-3’s action on microglia. This targeted approach could reduce the side effects associated with broader systemic treatments, further enhancing patient safety and treatment efficacy in Alzheimer’s management.
The Intersection of Cancer Therapies and Alzheimer’s
The application of cancer therapies, particularly those that involve immune modulation, has emerged as a groundbreaking strategy to tackle Alzheimer’s disease. Researchers have drawn parallels between cancer’s evasion of immune surveillance and Alzheimer’s disease’s ability to escape clearance by the immune system. By employing cancer treatment methodologies like checkpoint inhibition, scientists aim to harness the body’s immune system in a similar fashion to combat neurodegenerative diseases.
In this context, the use of TIM-3 antibodies – already established in oncology for enhancing anti-tumor immunity – is being repurposed to address Alzheimer’s. This cross-disciplinary approach not only illustrates the versatility of these immune-modulating strategies but also highlights the potential for repurposing existing drugs to expedite treatment availability for Alzheimer’s patients.
Cognitive Improvement through TIM-3 Modulation
Emerging studies have demonstrated that manipulating the TIM-3 pathway can lead to significant cognitive improvement in mouse models of Alzheimer’s disease. By inhibiting TIM-3, researchers found that not only was plaque clearance enhanced, but there were measurable gains in memory function as well. This dual benefit underscores TIM-3’s potential as a therapeutic target, offering hope for restoring cognitive abilities otherwise diminished by Alzheimer’s pathology.
Experiments measuring behavioral changes in these mouse models show that those with inhibited TIM-3 demonstrate improved navigation abilities in maze tests, indicative of enhanced memory retention and overall cognitive health. These findings suggest that treatments targeting the TIM-3 pathway could pave the way for novel therapeutic techniques that fundamentally change how Alzheimer’s is managed.
Current Research Trends in Alzheimer’s and Immune Therapy
As the research landscape evolves, the focus on immune therapy solutions for Alzheimer’s disease intensifies. A growing body of evidence supports the idea that immune checkpoints like TIM-3 play pivotal roles in preventing the brain’s immune cells from adequately responding to pathological conditions, specifically the buildup of amyloid plaques. This nexus of Alzheimer’s research and cancer therapies exemplifies how a collaborative and interdisciplinary approach can yield innovative treatment modalities.
Furthermore, ongoing studies aim to explore the genetic influences related to TIM-3 expression and its correlation with Alzheimer’s risk factors. Understanding these genetic determinants could unveil new biomarkers for early diagnosis, as well as guide the development and efficacy of forthcoming therapies targeting TIM-3 and similar immune pathways.
Focusing on Late-Onset Alzheimer’s and TIM-3
With late-onset Alzheimer’s accounting for a significant majority of cases, understanding TIM-3’s role in this context is critical. Genetic studies have identified polymorphisms in the TIM-3 gene as a risk factor for developing late-onset Alzheimer’s disease. This genetic linkage suggests that TIM-3 could not only serve as a therapeutic target but also a critical biomarker for identifying individuals at higher risk for developing Alzheimer’s.
In light of the genetic insights gathered, interventions designed to inhibit the action of TIM-3 may be timely for those in the early stages or at heightened risk for Alzheimer’s. By targeting this molecule, the potential exists to alter the course of the disease, steering treatment strategies towards not just symptomatic relief, but also toward preventative measures.
Future Directions for TIM-3 Therapy in Alzheimer’s Research
Looking ahead, the implications of TIM-3 therapy in Alzheimer’s research are profound. Experiments underway are not only validating the effectiveness of TIM-3 modulation in mouse models but are also preparing for clinical applications in human trials. The necessity of advancing animal research into human contexts is critical and underscores the urgency with which Alzheimer’s therapies must be developed.
Research teams are hopeful that their findings will facilitate the design of effective anti-TIM-3 therapies that can significantly reduce plaque load and restore cognitive functions, ultimately translating this research into tangible benefits for Alzheimer’s patients. The involvement of organizations like the National Institutes of Health further emphasizes the critical nature of this research and its potential impact on future Alzheimer’s treatment paradigms.
Role of Microglia in Alzheimer’s Disease and TIM-3
Microglia, as the brain’s resident immune cells, play a vital role in maintaining homeostasis and clearing cellular debris. In Alzheimer’s disease, the dysfunction of microglia due to the overexpression of inhibitory molecules like TIM-3 has significant repercussions, leading to the buildup of amyloid plaques which are thought to exacerbate neurodegeneration. Understanding the mechanisms by which TIM-3 alters microglial function provides insights into potential therapeutic interventions.
Studies focusing on microglial activation and TIM-3 inhibition suggest that enhancing microglial response could facilitate the reduction of plaque burden in the brain, thereby restoring some degree of normal cognitive function. This groundbreaking line of inquiry could provide a key to unlocking new treatment avenues for Alzheimer’s, representing a promising frontier in neuroimmunology.
Safety and Efficacy of TIM-3 Blockage in Human Trials
As research projects advance towards human trials, the safety and efficacy of TIM-3 blockage through antibodies represent a pivotal next step in the exploration of immunotherapy for Alzheimer’s disease. While preclinical studies in animals have shown promising results, it is critical to ensure that similar benefits translate to human subjects without adverse side effects. The thorough investigation into these therapies will provide a crucial foundation for developing new drugs aimed at managing Alzheimer’s disease effectively.
Moreover, the potential repurposing of existing anti-TIM-3 therapies from oncology into neurodegenerative contexts could streamline the development process and provide quicker access to treatment options for Alzheimer’s patients. Ensuring that these therapies undergo rigorous clinical testing will be essential in translating laboratory successes into clinical realities, ultimately aiming to improve patient outcomes in the face of this devastating disease.
Frequently Asked Questions
How is TIM-3 related to Alzheimer’s disease treatment?
TIM-3 is a checkpoint molecule that regulates the immune response in humans and has been identified as a genetic risk factor for late-onset Alzheimer’s disease. Research indicates that targeting TIM-3 can enhance the clearance of amyloid plaques in the brain, potentially leading to cognitive improvements in Alzheimer’s patients.
What role does TIM-3 play in the immune system therapy for Alzheimer’s?
In immune system therapy for Alzheimer’s, TIM-3 functions as an inhibitory molecule that prevents microglia from clearing amyloid beta plaques in the brain. By blocking TIM-3, researchers aim to reactivate microglia, allowing them to efficiently clear these harmful plaques and restore cognitive function.
Can TIM-3 therapies improve cognitive abilities in Alzheimer’s patients?
Preliminary studies in mice have shown that deleting the TIM-3 gene leads to improved memory and cognition by enabling microglia to clear brain plaques. While human trials are still needed, targeting TIM-3 could potentially translate to cognitive enhancements in Alzheimer’s treatment.
What discoveries about TIM-3 and Alzheimer’s were made in recent studies?
Recent studies have revealed that TIM-3 may inhibit microglial function, preventing them from attacking amyloid plaques, which are associated with Alzheimer’s. By understanding TIM-3’s role, researchers are hopeful that therapies targeting this molecule could create new treatment avenues for Alzheimer’s disease.
How does TIM-3 expression differ in Alzheimer’s patients compared to healthy individuals?
In Alzheimer’s patients, TIM-3 is expressed at significantly higher levels on microglia compared to healthy individuals. This overexpression inhibits microglial activity, hindering their ability to clear amyloid plaques, which are harmful in Alzheimer’s disease.
What are the potential implications of anti-TIM-3 antibodies in Alzheimer’s treatment?
Anti-TIM-3 antibodies have the potential to inhibit the negative effects of TIM-3, allowing microglia to effectively clear amyloid plaques in the brain. This approach could lead to new Alzheimer’s therapies that improve cognition and slow disease progression.
What are the challenges in developing TIM-3-based therapies for Alzheimer’s?
Challenges in developing TIM-3-based therapies for Alzheimer’s include ensuring targeted delivery of the therapy to the brain, understanding the complex interactions between TIM-3 and other immune mechanisms, and confirming the efficacy in human trials after successful preclinical studies.
How long has research on TIM-3 and Alzheimer’s been ongoing?
Research on TIM-3 and its implications for Alzheimer’s treatment has been ongoing for several years, with extensive studies taking about five years to yield significant findings regarding its role in microglial function and cognition.
What future studies are being planned regarding TIM-3 and Alzheimer’s disease?
Future studies aim to explore the effects of human anti-TIM-3 therapies in mouse models of Alzheimer’s, focusing on whether these therapies can effectively halt plaque development and improve cognitive functions associated with the disease.
| Key Aspect | Details |
|---|---|
| Research Focus | Studying TIM-3’s role in Alzheimer’s treatment. |
| Findings | Deleting TIM-3 expression in mice improved plaque clearance and cognitive function. |
| Mechanism | TIM-3 inhibits microglia, preventing them from clearing amyloid plaques. |
| Significance | Potential to repurpose anti-TIM-3 antibodies for human Alzheimer’s treatment. |
| Future Research | Testing human anti-TIM-3 in Alzheimer’s mouse models. |
Summary
TIM-3 and Alzheimer’s treatment are gaining traction as promising avenues for future research and therapy. This new study indicates that targeting the TIM-3 molecule could clear amyloid plaques in the brain, enhancing cognitive function in model organisms. The implications suggest a transformative approach to Alzheimer’s treatment, utilizing strategies proven effective in cancer therapy. As researchers continue to explore this innovative path, it could lead to significant advancements in managing or even treating Alzheimer’s disease.