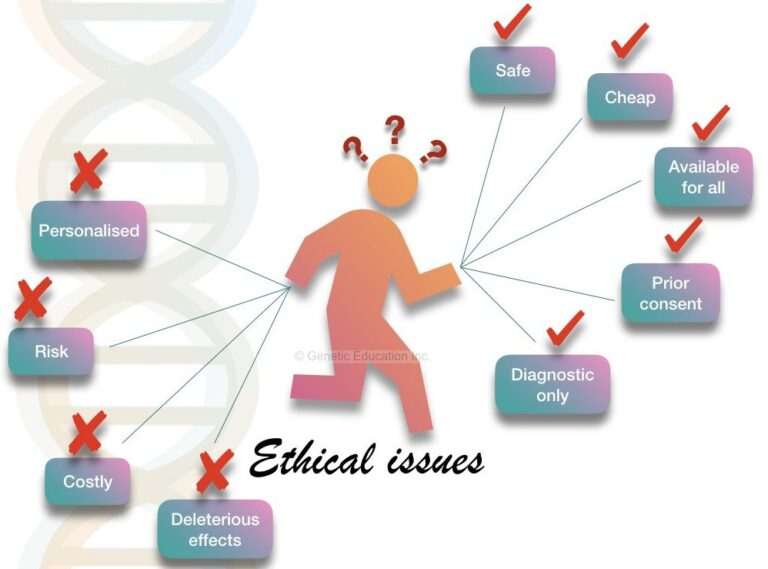
The ethical implications of CRISPR technology are becoming increasingly vital as gene editing advances to the forefront of medical science. This revolutionary technique not only holds the promise of potentially curing devastating conditions such as sickle cell anemia but also introduces profound questions surrounding gene editing ethics. As we explore the possibilities of altering human genetics, concerns about health equity and the risks associated with such interventions cannot be ignored. Who decides which traits are desirable, and how do we ensure fairness in access to these treatments? The conversation surrounding human variation in genetics adds complexity to the debate, emphasizing the need for a cautious and informed approach as we navigate the future of genetic modifications.
In recent years, the conversation surrounding gene editing techniques has gained prominence, significantly drawing attention to the moral dimensions involved. The scientific capabilities enabled by gene alteration tools present an exciting yet challenging landscape, where potential treatments for diseases like sickle cell anemia spark discussions over genetic ethics. Beyond just medical advancements, the implications for societal norms and individual rights raise urgent questions about equity in health care access and the potential risks inherent in manipulating the human genome. The dialogue also touches on philosophical considerations about human diversity and the definitions of what constitutes a healthy or ‘normal’ individual. As we delve into this complex arena of genetic intervention, the need for thorough ethical scrutiny remains paramount.
Understanding CRISPR Technology and Its Potential
CRISPR technology represents a breakthrough in gene editing by allowing precise modifications to DNA sequences, which scientists can leverage to address a range of genetic disorders. With CRISPR, researchers can remove, add, or alter sections of DNA with remarkable accuracy, making it a powerful tool in the fight against diseases like sickle cell anemia. For instance, by directly targeting the genes responsible for sickle cell disease, researchers can transform affected cells into healthy ones, potentially curing patients and alleviating suffering.
However, this remarkable potential comes with significant risks and ethical considerations. The ability to edit germline cells—that is, the eggs and sperm that contribute to a developing embryo—means changes could be passed down through generations, leading us to ponder the long-term consequences of ‘designer babies.’ Thus, while CRISPR technology offers promising solutions, we must carefully weigh its implications against the backdrop of unpredictable genetic outcomes.
Ethical Considerations in Gene Editing
One of the primary ethical concerns surrounding CRISPR technology is the notion of consent, particularly when editing genes in embryos. There’s an ongoing debate about who holds the authority to decide which genes should be altered and what criteria should govern those choices. Questions arise: Should parents have the right to select physical or cognitive traits for their children? And how might these decisions affect societal norms and values? These dilemmas highlight the need for ethical frameworks to guide the responsible use of gene-editing technologies.
Additionally, the disparity in access to gene editing raises crucial issues surrounding health equity. As Baer pointed out during his talk, while groundbreaking therapies might offer cures for diseases like sickle cell, the associated financial burden could create a chasm in access between affluent patients and those from underprivileged backgrounds. This leads to an urgent discourse on ensuring that advancements in medical science do not merely serve to widen existing inequities in healthcare.
CRISPR Ethical Implications: What Lies Ahead?
The implications of CRISPR technology extend beyond immediate medical outcomes and into the realm of social ethics. As we gain the capability to modify human DNA, society faces the question of what it means to be human. If certain traits can be edited or eliminated, how do we navigate the moral landscape that defines differences among individuals? Debates around whether genetic variations, such as those present in individuals with conditions like Down syndrome, should be edited or accepted speak to deeper societal values about diversity and acceptance.
Furthermore, ethical oversight is paramount to prevent misuse of CRISPR technology. The absence of stringent regulations can lead to unintended consequences, as seen in discussions about potential applications in military settings or for enhancing human performance. As we entertain the prospects of CRISPR technology, policymakers and bioethicists must collaborate to ensure that innovations are approached with caution, reflecting the diverse values and rights of future generations.
Health Equity in Gene Editing
Health equity is a critical aspect of the conversation surrounding gene editing technologies. The high costs associated with treatments like CRISPR for sickle cell anemia present a stark reality: while some can afford life-changing therapies, many may be left without access due to economic barriers. The challenge lies in creating frameworks that ensure all populations can benefit from advances in gene editing, rather than perpetuating cycles of inequality based on socioeconomic status.
Moreover, discussions around health equity must address the ethical responsibility of the scientific community. As researchers continue to push the boundaries of gene editing, they must advocate for policies that prioritize inclusivity in healthcare decision-making. This includes engaging with various stakeholders, including advocates for marginalized groups, to ensure that advancements do not exacerbate existing disparities but rather contribute to a more just health system.
The Role of Genetics in Human Variation
Human genetics exhibit a fascinating and complex variation that underscores the natural diversity within our species. This variation can influence a multitude of traits and health outcomes, posing both challenges and opportunities for gene editing. As researchers navigate these uncharted waters, understanding that not all genetic traits signify problems is vital. Genetic differences, such as those seen in individuals with albinism or deafness, challenge the normative medical narrative that equates health solely with the absence of disease.
By recognizing and respecting human variation, we open the door to a more inclusive perspective on gene editing. This perspective dilutes the pressure to ‘normalize’ traits that are often pathologized and instead embraces the concept of human diversity as a strength. It prompts a reconsideration of how gene editing might serve to enhance what already exists rather than solely correcting perceived ‘deficiencies’—reinforcing the need for an ethical framework to guide scientific advancements.
Challenges of Germline Editing
Germline editing, which involves altering genes in the embryo, presents a particularly contentious frontier in genetic research. While it holds the promise of eradicating hereditary diseases before they manifest, it raises profound ethical questions regarding the nature of human intervention in evolution. The modification of an embryo could potentially reshape not just one life, but generations to come, leading us to ponder the boundaries of what is acceptable in scientific exploration.
Moreover, the unpredictability of gene interactions amplifies the risks associated with germline editing. As Baer pointed out, the complexity of genes means they do not function in isolation; they are part of intricate networks that influence various biological processes. This interconnectedness complicates our understanding of potential side effects and unintended consequences, highlighting the importance of rigorous research and ethical discourse before committing to such profound changes.
Societal Perspectives on Genetic Modification
As CRISPR technology advances, societal perspectives on genetic modification become increasingly critical. The public’s understanding and acceptance of gene editing practices must be developed through education and open conversation. Fostering a societal discourse that considers the diverse views on disease, wellness, and human enhancement can help mitigate fears and resistance while promoting informed decision-making in scientific applications.
Additionally, the role of media cannot be underestimated in shaping public perceptions of gene editing. As dramatized narratives in television intersect with scientific realities, they have the power to influence opinions and ethical judgments about genetic technologies. It is essential for creators, scientists, and ethicists to collaborate in conveying accurate, balanced information that fosters both curiosity and caution concerning the advances in genetic modification.
Regulation and Oversight in Gene Editing
The regulation of gene editing technologies is pivotal for ensuring ethical practices in research and clinical applications. As CRISPR technology becomes more widely integrated into medical practices, the establishment of comprehensive oversight mechanisms is crucial. These regulations should encompass considerations such as safety, efficacy, and ethical implications of gene editing, aiming to protect individuals and communities from potential misuse or unintended consequences.
International cooperation is also needed to address the global nature of gene editing advancements. Since regulations can differ significantly across countries, a harmonized approach might be necessary to prevent unethical practices, such as unauthorized germline modifications. By advocating for responsible innovation on a global scale, we can work towards a future where gene editing not only offers medical solutions but also reflects our shared ethical values.
Future Directions in Gene Editing Research
The future of gene editing technology like CRISPR is filled with both promise and uncertainty. As researchers continue to explore the potential of gene editing in combatting genetic disorders, it’s essential to remain vigilant about the ethical arguments that accompany these advancements. Encouraging responsible research practices, coupled with ongoing dialogue about health equity and societal impacts, will be key to navigating the next stages of this rapidly evolving field.
Moreover, as scientific understanding deepens, novel techniques may arise that address some of the current limitations of CRISPR technology, such as off-target effects. Advancements that prioritize safety and precision can enhance the acceptability of gene editing within society. Ultimately, a multidisciplinary approach combining insights from genetics, ethics, and patient advocacy will be vital in shaping the responsible use of gene editing technologies for generations to come.
Frequently Asked Questions
What are the ethical implications of CRISPR technology in gene editing?
CRISPR technology presents various ethical implications, particularly concerning the manipulation of human genetics. The ability to edit genes raises questions about the morality of altering traits, potential discrimination based on genetic outcomes, and the long-term effects on human variation. Ethical debates focus on who makes these decisions and the effects of such changes on health equity.
How does gene editing ethics relate to sickle cell anemia gene therapy?
Gene editing ethics are significantly highlighted in sickle cell anemia gene therapy discussions, as CRISPR offers potential cures but also raises concerns about access, affordability, and social justice. Treatments like these could deepen existing inequalities in healthcare if only accessible to certain populations.
What are the potential risks associated with CRISPR technology?
The potential risks associated with CRISPR technology include unintended genetic changes, which could lead to health problems, and ethical concerns regarding germline editing. Risks also arise from the possibility of exacerbating health inequalities and making medical decisions that may not align with individual or societal values.
How does health equity relate to CRISPR and gene editing?
Health equity is a central concern in discussions about CRISPR and gene editing, particularly regarding who can access these advanced treatments. There is a fear that as gene editing technologies advance, they may only benefit affluent populations, leaving marginalized communities at risk and widening the health gap.
What ethical questions arise when considering gene editing for human variation?
Ethical questions surrounding gene editing for human variation focus on the definition of ‘normalcy’ and the implications of genetically altering traits that are part of human diversity. This raises debates about parental rights to modify genetic traits of children and the societal impacts of defining certain genetic conditions as pathologies.
| Key Point | Details |
|---|---|
| CRISPR Technology Overview | CRISPR allows precise editing of both somatic and germline genes, potentially curing genetic diseases. |
| Ethical Questions | Challenges arise over the right to alter human attributes and the decision-making process behind such changes. |
| Cost and Accessibility | Treatments like the sickle cell cure cost over $2 million, raising issues about who can access it. |
| Health Equity | Innovations can exacerbate disparities, impacting marginalized communities more negatively. |
| Oversight and Regulation | There are concerns about the lack of regulation on gene editing practices worldwide, particularly in countries with less oversight. |
| Unintended Consequences | Gene editing could lead to unforeseen health issues due to the complex nature of gene interactions. |
Summary
CRISPR ethical implications are at the forefront of discussions about gene editing technology. As scientists gain the ability to edit human DNA, the ethical dilemmas surrounding the consequences of such actions intensify. The potential for curing genetic diseases is juxtaposed with questions about identity, equity, and the moral responsibilities of parents and society. With costs making treatments accessible only to a few and the risks of unintended effects highlighted, the discourse emphasizes the need for careful consideration and regulation in the face of rapidly advancing scientific possibilities.