Medical research funding cuts pose a significant threat to advancements in healthcare and patient safety. Recently, the freeze on over $2 billion in federal grants to institutions like Harvard has cast a shadow on efforts that prioritize the welfare of participants in clinical studies. The abrupt halt in funding not only disrupts ongoing projects but also undermines the integrity of research overseen by Institutional Review Boards (IRBs) that ensure ethical guidelines are followed in clinical trials. As studies are forced to pause or abandon crucial participant safety measures, the long-term implications on research integrity and federal research funding cannot be overlooked. In a landscape where the impact of funding cuts on research is increasingly evident, stakeholders must recognize the crucial balance between ethical oversight and the resources needed to uphold patient safety in clinical research.
The recent downturn in medical funding is not merely a budget issue; it represents a critical juncture for research ethics and the integrity of clinical trials in the United States. As funding streams dry up, the ramifications extend beyond financial constraints, jeopardizing essential oversight mechanisms that protect vulnerable study participants. Oversight committees, such as Institutional Review Boards (IRBs), are fundamental in ensuring that research protocols adhere to ethical standards and that participant welfare remains a priority. The tightening of budgets could thwart collaborative research efforts essential for breakthroughs in treatments and interventions. As discussions around financial implications in the medical research sector unfold, it’s imperative to explore how these funding challenges affect not only researchers but also, crucially, the patients whose safety hangs in the balance.
The Impact of Funding Cuts on Medical Research Quality
Medical research funding cuts significantly compromise the integrity and quality of research conducted across various institutions. When funding is constricted, research trails suffer from a lack of necessary resources, leading to delays in study timelines and, ultimately, impaired patient safety. The ability to conduct thorough reviews and oversight by institutional review boards (IRBs) is also jeopardized, as these boards rely on adequate funding to carry out their crucial roles effectively. Without sufficient financial support, the capacity to monitor and ensure ethical compliance becomes severely limited, which could adversely affect patient outcomes in clinical trials.
Moreover, substantial cuts in funding directly influence the recruitment process for research studies, as fewer resources are available for participant engagement and retention strategies. This could lead to fewer volunteers wanting to participate in clinical trials, creating a skewed demographic and unrepresentative data inputs. This has lasting repercussions on the generalizability of the research findings. As studies face interruptions or cancellations due to funding shortages, the critical need for continuous and stable financial backing becomes more evident in maintaining the highest standards of medical research.
Patient Safety in Clinical Trials During Funding Cuts
In the face of funding cuts, patient safety in clinical trials is potentially put at risk due to halted oversight and diminished resources typically allocated for monitoring participant well-being. The system of ethical checks and balances provided by IRBs is essential for safeguarding the interests of participants. When financial constraints limit the operational capacity of these boards, the ability to respond to adverse events or conduct ongoing risk assessments diminishes. Thus, patient security within clinical settings becomes compromised, increasing the likelihood of negative outcomes.
Furthermore, the presence of an IRB in clinical research plays a pivotal role in ensuring that informed consent processes are transparent and that participants are aware of their rights. Funding cuts can impair training programs for researchers on ethical issues, leading to lapses in proper guidance when interacting with trial participants. This, in turn, places participants in vulnerable positions where their understanding of the research and associated risks is diminished, negatively affecting their autonomy and well-being.
The Role of Institutional Review Boards (IRBs) in Research
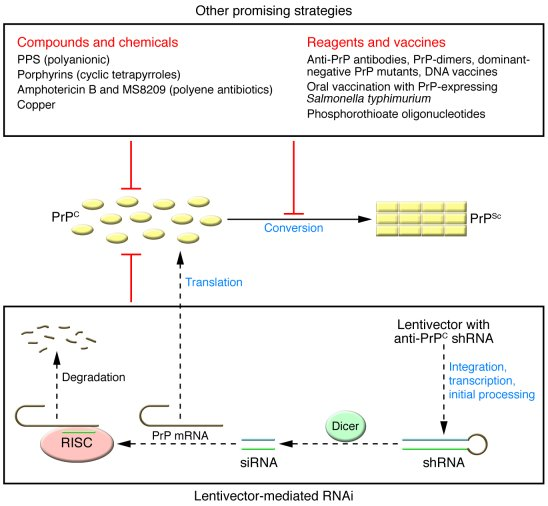
IRBs act as essential watchdogs within the research ecosystem, safeguarding the rights of human subjects throughout the duration of a clinical study. Their independence and the rigorous ethical standards they uphold ensure that research involving human participants remains grounded in respect for their dignity and rights. As funding cuts emerge, there’s a direct impact on the resources available for these boards to function effectively, including their ability to conduct independent reviews and provide meaningful oversight. This could lead to ethical lapses that may affect study outcomes and the welfare of the participants.
Moreover, the increasing reliance on single IRBs (sIRBs) for multisite research due to funding restrictions can increase pressure on these entities, which may already be stretched thin. The implication is clear: when funding is inadequate, the capacity for thorough ethical reviews may dwindle, raising concerns over the monitoring of participant safety and welfare, and potentially undermining public trust in the research process.
Reinforcing Research Ethics and Integrity Amidst Financial Constraints
The ethical conduct of research is at risk in an environment marked by funding cuts, which can lead to increased pressures on researchers to produce results with limited resources. This may foster an environment where ethical boundaries are pushed, compromising integrity in the pursuit of funding or results. Institutions may struggle to uphold ethical standards without the necessary financial support for compliance training and ethical oversight provided by IRBs.
Furthermore, diminished funding may limit researchers’ ability to engage thoroughly with the communities they serve, impacting the ethical frameworks within which they operate. Effective ethical oversight and consistent adherence to established guidelines are essential for maintaining credible research practices and patient safety. Therefore, financial support for ethical practices must remain a priority, even in times of limited funding, to ensure that researchers not only meet regulatory requirements but also uphold the fundamental rights and welfare of research participants.
Federal Research Funding Implications for Medical Innovation
Federal research funding plays a vital role in driving the advancement of medical innovations that can save lives and improve health outcomes across populations. With the recent cuts in funding, the implications for medical research innovation are profound. Research initiatives aimed at developing treatments for chronic diseases like Alzheimer’s or cancer may face significant delays or even cancellations, stalling progress that thousands of patients rely upon.
Moreover, federal funding cuts can hinder collaborative efforts between institutions, often necessary for large-scale trials that yield comprehensive data. By disrupting these collaborations, the cuts limit the sharing of resources and knowledge that accelerate innovation, further stifling breakthroughs in medical therapy. As institutions scramble to adapt to financial constraints, the long-term consequences for health advancements and patient care can be dire.
Navigating Challenges of Patient Recruitment Post-Cuts
Patient recruitment becomes increasingly challenging in the wake of funding cuts, especially when resources for outreach and participant engagement are critical. Research studies frequently depend on these funds to educate potential participants and to inform them about the benefits and risks of enrolling in trials. With diminished funding, outreach efforts may be severely curtailed, leading to fewer volunteers, which directly hampers the ability to conduct studies that are representative of the wider population.
Furthermore, without adequate financial backing, participants might perceive clinical trials as less trustworthy or less beneficial, leading to a potential decline in public trust in research studies. This erosion of trust can be detrimental to future studies, creating a cycle where fewer participants are willing to engage, compounding the impacts of already reduced research funding.
The Future of Collaborative Research in a Post-Cut Environment
The landscape of collaborative research faces substantial challenges in the aftermath of funding cuts, particularly when multiple institutions rely on shared resources and IRB oversight for large-scale studies. Many research initiatives that would typically involve various academic or medical institutions might face interruptions, which can lead to lost opportunities for innovation. The complexity of coordinating among multiple stakeholders becomes increasingly difficult without the foundational support of adequate funding.
Additionally, collaborative research hinges on the ability to navigate various regulatory and operational hurdles, which require continuous oversight from funded IRBs. The introduction of mechanisms like SMART IRB aimed at strengthening collaboration effectively may falter under financial strain, ultimately leading to a broken system where innovative solutions to critical public health issues cannot be realized.
Advocating for Sustainable Medical Research Funding
The need for advocacy around sustainable medical research funding has never been more pronounced, especially in light of recent funding cuts that threaten the integrity of clinical trials and the health of participants. Stakeholders, including researchers, healthcare professionals, and patient advocates, must come together to emphasize the importance of long-term funding for maintaining ethical standards and ensuring the safety of participants in research. Public awareness campaigns can be vital in highlighting the consequences of funding cuts and rallying support for robust funding mechanisms.
Furthermore, policymakers should be encouraged to recognize the value of investing in research as part of a broader public health strategy. Sustainable funding not only drives innovation but ensures a commitment to patient safety, ethical oversight, and community trust in research outcomes. By advocating for a stable funding environment, the health of future generations can be safeguarded, maintaining a focus on promoting health equity and advancing medical knowledge.
Long-term Consequences of Funding Cuts on Healthcare Delivery
As funding cuts ripple through the medical research ecosystem, the long-term impact on healthcare delivery could be significant. A decline in research activity may stymie the development of new treatments and innovations that improve patient care and outcomes. The repercussions can extend beyond research settings, affecting clinical practices and ultimately leading to a decline in the quality of healthcare services available to patients.
Lack of funding may also reinforce existing disparities within healthcare systems as researchers may prioritize studies that receive funding over those that address critical health issues in underserved populations. If funding sources remain inconsistent, the overall responsiveness to patient needs will likely diminish, further exacerbating healthcare inequities and limiting access to essential services. Thus, addressing funding cuts is crucial not only for the integrity of research but also for ensuring equitable healthcare delivery.
Frequently Asked Questions
How do medical research funding cuts impact patient safety in clinical trials?
Medical research funding cuts can severely compromise patient safety in clinical trials by halting ongoing studies and delaying vital research. Without adequate funding, institutional review boards (IRBs) may lack the necessary resources to operate effectively, undermining their role in overseeing research and safeguarding participant welfare.
What are the implications of funding cuts for the ethical conduct of medical research?
Funding cuts can threaten research ethics and integrity by limiting oversight capabilities of IRBs. This could lead to lapses in ethical review processes, ultimately risking the health and rights of participants and raising concerns about transparency in research endeavors.
How does the IRB role in medical research get affected by funding cuts?
Cuts in medical research funding result in constraints on IRB resources and staffing. This can hinder their ability to provide thorough reviews of research proposals, leading to potential gaps in participant protection and an increase in ethically questionable practices within clinical trials.
Why is the impact of funding cuts on research significant for future medical advances?
The impact of funding cuts on research is significant as it can create delays or cancellations in promising studies, impeding innovation in medical treatments. Without sufficient financial support, collaborative efforts among multiple sites may falter, restricting the ability to advance medical knowledge and improve patient outcomes.
What are the federal research funding implications of the recent funding cuts?
The recent funding cuts disrupt the federal research funding landscape, jeopardizing grants essential for vital research. This situation can lead to lost opportunities for groundbreaking discoveries and a decrease in the overall quality and availability of medical research, ultimately affecting patient care and safety.
| Key Point | Details |
|---|---|
| Funding Freeze | The Trump administration halted over $2 billion in federal research grants to Harvard, affecting patient safety efforts. |
| Impact on the SMART IRB | The stop-work order has disrupted the SMART IRB, which oversees medical research at multiple sites, essential for patient safety. |
| Role of Institutional Review Boards (IRBs) | IRBs ensure compliance with laws and protect the rights and welfare of research participants. |
| Importance of IRB in Research | IRBs serve as checks and balances in medical research to prevent harm and ensure ethical standards. |
| Long-term Effects of Cuts | Funding cuts lead to halted studies, increased public mistrust, and potential risks to patient safety. |
| Historical Context | Past medical experiments highlight the need for stringent oversight and ethical protocols in research. |
Summary
Medical research funding cuts pose a significant threat to the integrity and safety of clinical trials. The recent halt in federal research funding not only disrupts ongoing studies but also jeopardizes the rights and welfare of patients participating in these critical research efforts. As funding becomes scarce, the risk of compromised oversight increases, heightening public skepticism and potentially leading to harm for study participants. Maintaining robust funding is essential to uphold ethical standards and ensure that medical research continues to safeguard and benefit the community.